Publié sur :
ECCO 1999 - Vienna, Austria - 2000
Voir la version PDF de cette publication :

Multicentric randomized trials for high grade osteogenic osteosarcoma. Cost-Effectiveness ?
S. Alkallaf, A. Tabbi, B. Markowska, H. Cornille, G. Delepine,
Nicole Delepine
Multicentric randomized trials for high grade osteogenic osteosarcoma. Cost-Effectiveness ?
Backgrounds
Randomization of treatment represents the best theoretical way to avoid bias when performing studies between two groups of patients.
Evidence based medicine ?
Unfortunately for many doctors, this rule of randomization leads to forget the other methodological and ethical aspects of clinical research on human beings and consists in an intellectual "terrorism".
Each non randomized trial is, a priori, doubtful.
The publications, which relate the results of pilot studies are discriminated or refused in American reviews.
It's the same for communications.
Question
Are multicentric randomized trials really efficent in clinical practice for our patients ?
And the scientific progress ?
Which are their cost for our patients ?
And for social insurance systems ?
Aim of the study
This study of the last 20 years tries to compare the benefice /cost of randomized trials in high grade osteosarcoma to those of monocentric studies in term of disease free survival for patients and cost effectiveness for the community.
Introduction
These last 20 years chemotherapy of patients with osteosarcoma has been dramatically improved. Nevertheless dilemmas and controversies have been continuously developed.
Main controversies
1°) Is chemotherapy usefull for patients with high grade osteosarcoma?
2°) Is limb salvage dangerous for patients?
3°) Is methotrexate dose and/or pharmacokinetics a pronostic factor?
Search method
All published treatment studies about osteosarcoma have been detected.
By a double computed search (Medline and Cancernet),
By the analysis of literature of published data,
Completed by the analysis of main orthopaedic and oncologic congresses abstracts.
Criteria for inclusion
After computed search of all published studies on treatment of osteosarcoma, we analyzed all studies including more than 20 patients in each treatment arm with at least 5 years follow up and clear conclusions.
Included studies
Multicentric randomized trials
Randomized monocentric trials
Monocentric pilot studies
when satisfying inclusion criteria
Nowaday established data
1. Chemotherapy is useful for high grade osteosarcoma
2. Conservative surgery is not dangerous when
3. Use of MTX gives better results
Evaluation
Published conclusions were compared to actual state of the art.
Useful trials (giving the right conclusions) were rated from +1 to +10 following their publication rank.
Misleading conclusions of trials were rated - 1 to -10 following their potential consequences.
This scoring system has been applied :
To the treatment conclusions with direct benefice to the patient
To others conclusions useful to the knowledge of the illness.
RESULTS
The analysis of the literature demonstrates the following facts :
Observation 1
Multicentric trials never reproduce completely the good results of pilot studies that led to them !
Observation 2
No multicentric randomized trial has permitted to point out any factor able to improve the lenghth or/and the quality of life of patients with osteosarcoma.
The first advocates of chemotherapy for osteosarcoma
Cortes N. Eng. J. Med. 1974
Jaffe N. Eng. J. Med. 1974
Sutow J. Bone Joint Surg. 1976
Rosen Cancer 1976
Frei J. Nat. Canc. Inst. 1978
Bacci J. Bone Joint Surg. 1980
Bleyer J. Bone Joint Surg. 1980
All the studies (excepted study of Frei) were monocentric and non randomized...
In 1979 Jaffe and Rosen strongly advocated adjuvant chemotherapy.
Historical comparaison was demonstrative enough.
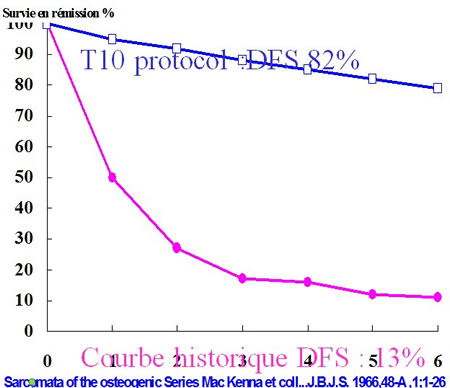
Any trial chemotherapy versus nothing would be useless and unethical.
Controversy of 1980
People who advocated randomized trials among them the Mayo Clinic and the National Cancer Institute replied that a spontaneous amelioration of the ilness and a stage migration could explain the difference.
They critisized the Rosen methodology and the use of historical comparaison.
Detractors of chemotherapy in osteosarcoma
Rosenberg C. - Treatment Rep. 1979 - 39 patients
Jasmin - GETO-EORTC trial - 1979 - 8 centers 27 patients randomized
Edmonson J. - Clin. Oncol. - 1984 - Mayo Clinic 38 patients randomized
(led to randomized trial MIOS 1984 (published by Link 1986)
Taylor J. - Nat. Canc. Inst. - 1989 - 9 centers 350 patients
Results of MIOS trial
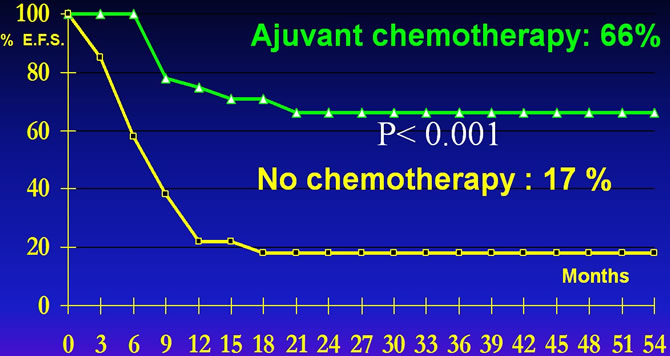
Adjuvant chemotherapy increases by 4 times the event free survival.
LinkM.P. The effectof adjuvant chemotherapy on relapse free survival New Eng J M 1986, 314:160
Consequences for patients to have been randomized in MIOS Trial (1)
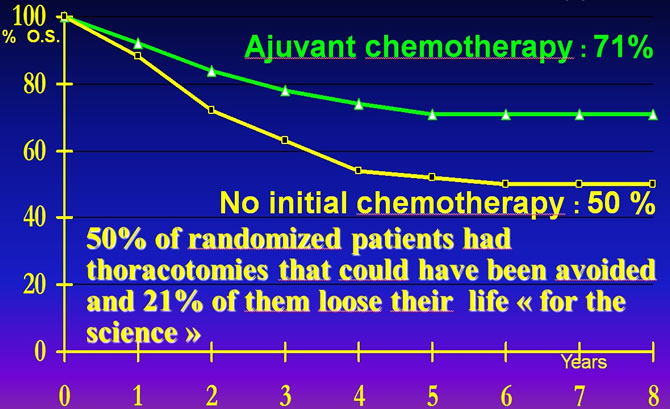 Link M.P. The effectof adjuvant chemotherapy on relapse free survival New Eng J M 1986,314:160
Link M.P. The effectof adjuvant chemotherapy on relapse free survival New Eng J M 1986,314:160
Critical Review of litterature : Cost for U.S. patients of the randomized MIOS Trial
1°) 50% of randomized patients had thoracotomies that could have been avoided and 21% of them loose their life "for the science".
2°) The randomized control of efficacy of chemotherapy delayed its application by 6 years 1979-1986.
3°) Resulting in secondary death from suboptimal therapy for about 4000 patients.
Interest of the pre trial controversy
The open controversy which arose before the MIOS trial permited to patients to obtain a better information and to choose their treatment if they wanted.
Among the 77 patients who refused to be randomized (77%) choosed adjuvant chemotherapy arm.
Demonstrating that informed patients can be more Wise than doctors !
First advocates of modern conservative surgery :
Marcove et Rosen, Cancer, 1979
Delepine, RCO, 1985
Goorin, JCO, 1987
Eilber, JCO, 1987
Sprinfield, J.B.J.S., 1988
none of these works were neither randomized, nor multicentric.
Detractors of limb salvage
Coss 80 conclusion :
"Amputation and foot's reversal improve the survival in complete remission more than resection reconstruction with prosthesis".
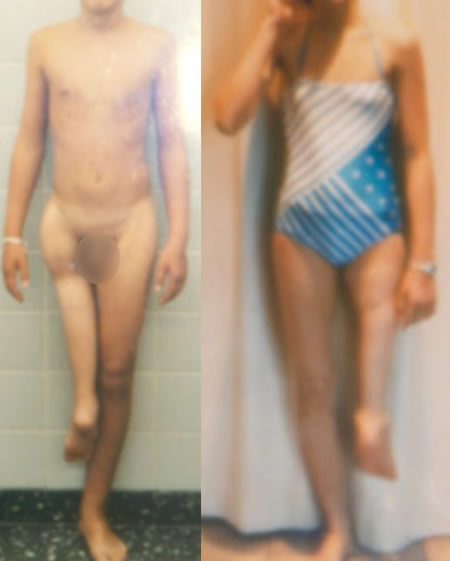 Winkler COSS 80 JCO 1986 : Multicentric randomized trial describing data from 34 different centers.
Winkler COSS 80 JCO 1986 : Multicentric randomized trial describing data from 34 different centers.
Résultat 1986 du protocole COSS 80
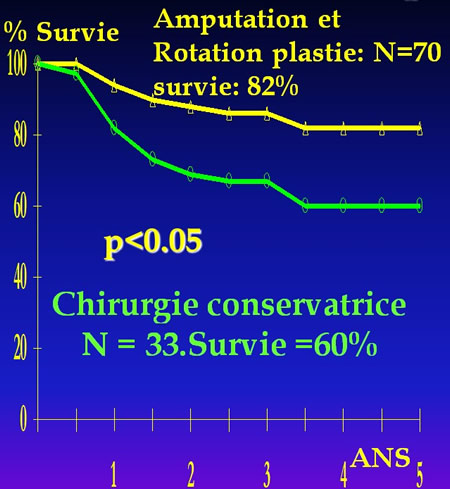
La publication en 1979 du protocole multicentrique du Cancer and Leukemia Group B * et en 1986 de l'essai multicentrique COSS 80 ** ont mis en évidence une diminution significative (p<0.05) de l'espérance de guérison chez les malades porteur de grosses tumeurs et traités par chirurgie conservatrice.
*Cortes Adjuvant therapy of operable osteosarcoma-Cancer and Leukemia Group B Experience Recent Results in Cancer Research 1979;68:16-24
**Winkler K. Einfluss des lokal chirurgischen Vorgehens auf die Inzidenz von Metastan nach neo-adjuvanter Chemotherapie des Osteosarkoms.Zeitschrift fur Orthopaedie und Grenzgebiet1986
Sloan Kettering Cancer Center Experience New York
Mutilating surgery does not improve disease free survival of patients.
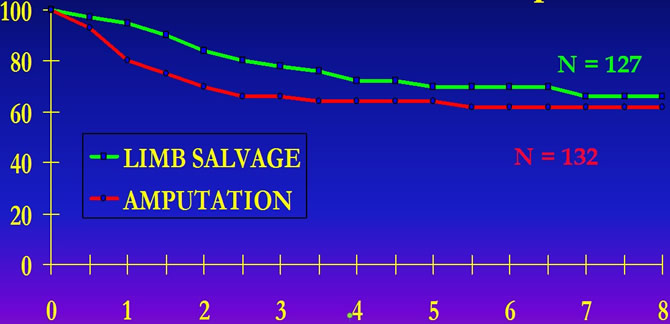 MEYER and all."CHEMOTHERAPY FOR NON METASTATIC OSTEOGENIC OSTEOSARCOMA: THE MEMORIAL S.K.C.C.EXPERIENCE " J.Clin.Oncol.10,1,5-15 , 1992
MEYER and all."CHEMOTHERAPY FOR NON METASTATIC OSTEOGENIC OSTEOSARCOMA: THE MEMORIAL S.K.C.C.EXPERIENCE " J.Clin.Oncol.10,1,5-15 , 1992
Florida University Experience
Mutilating surgery does not improve disease free survival of patients.
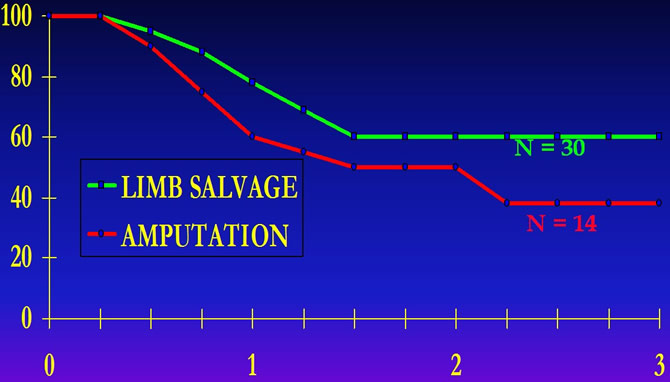 Graham Pole J. and all."Neoadjuvant chemotherapy for patients with osteosarcoma : University of Florida studies" in B.Humphrey ed.1993 Kluwer Ac.Publis.
Graham Pole J. and all."Neoadjuvant chemotherapy for patients with osteosarcoma : University of Florida studies" in B.Humphrey ed.1993 Kluwer Ac.Publis.
UCLA Experience
Mutilating surgery does not improve disease free survival of patients.
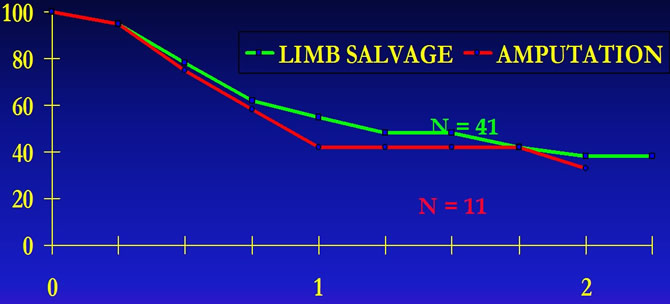 ECKARDT J. J. and all "Management of stage I I B osteogenic sarcoma : Experience at the university of california, Los Angeles" Cancer Treat.Symp 3 :117-130 ,1985.
ECKARDT J. J. and all "Management of stage I I B osteogenic sarcoma : Experience at the university of california, Los Angeles" Cancer Treat.Symp 3 :117-130 ,1985.
American multicentric study
Mutilating surgery does not improve disease free survival of patients.
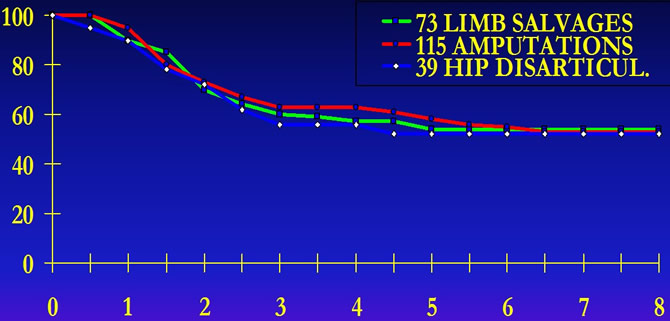 SIMON M.A.and all "Limb salvage treatment versus amputation for osteosarcoma of the distal end of the femur" J.Bone Joint Surg. 68A,9:1331-1338, 1986
SIMON M.A.and all "Limb salvage treatment versus amputation for osteosarcoma of the distal end of the femur" J.Bone Joint Surg. 68A,9:1331-1338, 1986
Rosen Rules for osteosarcoma
1°) Preoperative chemotherapy is an investigational method not a recipe.
2°) Give at least 240 grammes / m² de MTX.
3°) Do not give too much liquid after MTX.
4°) Never delay the MTX curses.3
5°) Increase the doses if response is incertain.
6°) Increase the doses if si serum peakis insuffisant (H4> 1500 µmol/L) (Rule added since 1995).
Critical Review of litterature : State of the art in 2001
Nevertheless multicentric groups still discuss the efficacy of Rosen scheme and pretend to obtain "non statistically different results with easier protocols".
Preoperative chemotherapy : an investigational method not a recipe
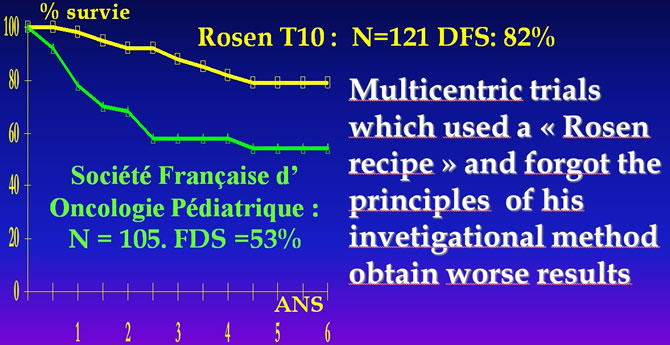 Rosen G, Marcove Nirenberg Chemotherapy forOsteogenic Sarcoma: An Investigative Method not a Recipe Cancer Treat.Resp.1982;9;1687-1697. M.BRUNAT-MENTIGNY "La reproduction du protocole de ROSEN pour les ostéosarcomes. Bull.Cancer 1988, 75:201-206.
"At least 240 grammes / m²"
Rosen G, Marcove Nirenberg Chemotherapy forOsteogenic Sarcoma: An Investigative Method not a Recipe Cancer Treat.Resp.1982;9;1687-1697. M.BRUNAT-MENTIGNY "La reproduction du protocole de ROSEN pour les ostéosarcomes. Bull.Cancer 1988, 75:201-206.
"At least 240 grammes / m²"
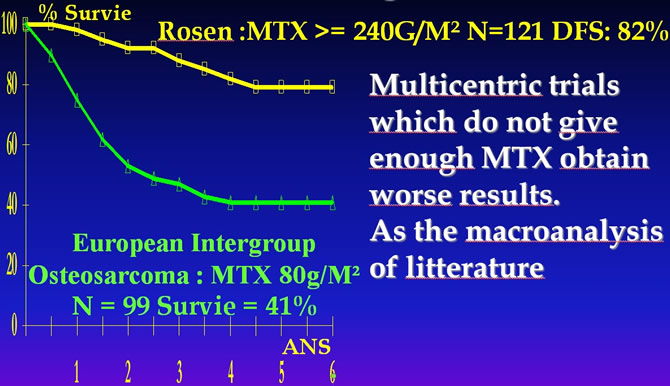 Rosen G, Marcove RC, Caparros B et al. Primary osteogenic sarcoma. The rationale for preoperative chemotherapy and delayed surgery. Cancer, 1979 ; 43 : 2163-77. Bramwellet all A Comparaison of two short Chemotherapy Regimen in operable Osteosarcoma of Limbs in Children and Young Adults : The first Study of the European Osteosarcoma Intergroup J.Clin.Oncol.1992 10:1579-1591
Rosen G, Marcove RC, Caparros B et al. Primary osteogenic sarcoma. The rationale for preoperative chemotherapy and delayed surgery. Cancer, 1979 ; 43 : 2163-77. Bramwellet all A Comparaison of two short Chemotherapy Regimen in operable Osteosarcoma of Limbs in Children and Young Adults : The first Study of the European Osteosarcoma Intergroup J.Clin.Oncol.1992 10:1579-1591
"Do not give too much liquid after MTX"
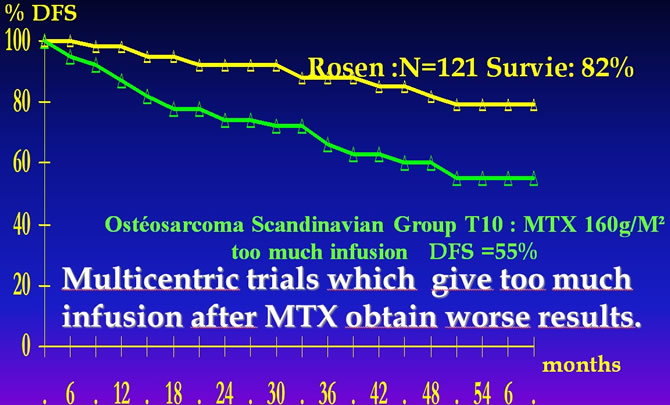 Rosen G, Marcove RC, Caparros B et al. Primary osteogenic sarcoma. The rationale for preoperative chemotherapy and delayed surgery. Cancer, 1979 ; 43 : 2163-77.Saeter G.Treatment of osteosarcoma of the extremities with emphasis of the effects of preoperative Chemotherapy .J.Clin.Oncol.1991;9;1766-1775
Rosen G, Marcove RC, Caparros B et al. Primary osteogenic sarcoma. The rationale for preoperative chemotherapy and delayed surgery. Cancer, 1979 ; 43 : 2163-77.Saeter G.Treatment of osteosarcoma of the extremities with emphasis of the effects of preoperative Chemotherapy .J.Clin.Oncol.1991;9;1766-1775
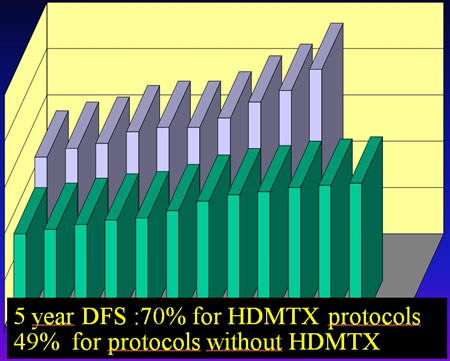
Litterature : Résults of neoadjuvant protocols
Protocols which do not give HDMTX decrease the disease free survival of patients by 20%.
Correlation DFS/Dose Intensity of MTX
The crucial importance of Doses of MTX is obvious when we analized the results of all published trials.
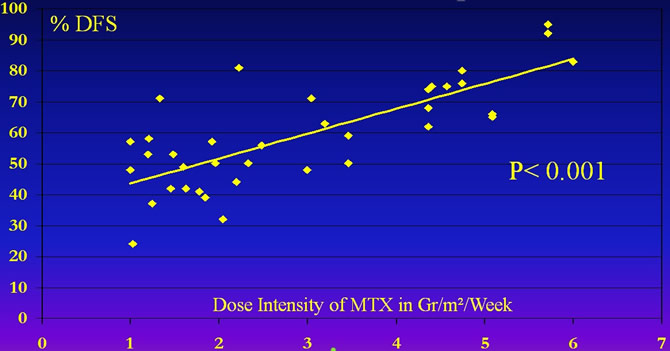 Delepine Nicole. Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. A literature analysis, about 1909 cases. Cancer, 1996, 78 : 2127-35.
Delepine Nicole. Influence of methotrexate dose intensity on outcome of patients with high grade osteogenic osteosarcoma. A literature analysis, about 1909 cases. Cancer, 1996, 78 : 2127-35.
T10 RESULT FOR NON METASTATIC OSTEOSARCOMA OF THE EXTREMITIES
With 15 years follow up the Rosen's protocols reviewed by independant investigators remain the gold standart.
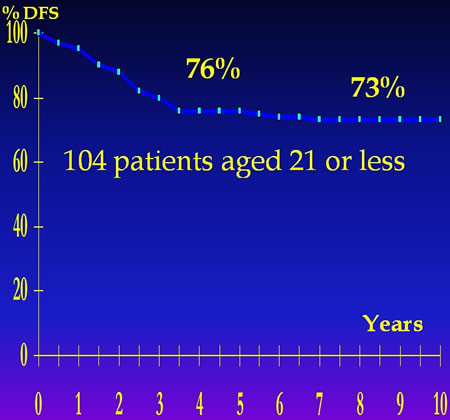 MEYER and all."CHEMOTHERAPY FOR NON METASTATIC OSTEOGENIC OSTEOSARCOMA: THE MEMORIAL S.K.C.C.EXPERIENCE" J.Clin.Oncol. 10,1,5-15,1992
MEYER and all."CHEMOTHERAPY FOR NON METASTATIC OSTEOGENIC OSTEOSARCOMA: THE MEMORIAL S.K.C.C.EXPERIENCE" J.Clin.Oncol. 10,1,5-15,1992
Promoters of chemotherapie's intensification
Rosen, 1976, Cancer
Jaffe, 1977, Cancer (weekly Methotrexate)
Cortes, 1978, R.R.C.R. (full dose of Adriamycin)
Rosen, 1979, Cancer, (achieved by neoadjuvant chemotherapy and individualisation)
Bacci, 1980, J.B.J.C. (HDMTX > MTX)
Rosen, 1982, Cancer
These who doubted of the importance of methotrexate's dose
Krailo, CCG741, 1987, Med. Ped. Onc.
"Dose of Methotrexate of 690 mg by course led to the same results !".
Burgers, EI080831, 1992, JCO
"ADR-CDDP lead to better results than ADR-CDDP-MTX".
Craft, EI080861, 1996, "ADR-CDDP lead to the some result than a more complex protocol including methotrexate."
these trials didn't respect the most important backgrounds of Rosen's protocol :
- delay between 2 courses of methotrexate,
- individualization of methotrexate dose upon evaluation of clinical efficacy,
- too large hydration of patients receiving methotrexate too long preoperative phase,
- too low numbers of methotrexate courses,
- etc...
Furthermore, independent evaluation of T7 and T10 demonstrates, 15 years later, that these protocols remain the most effective in osteosarcoma disease free survival at 10 years.
W.H. Meyer, M.D. Link, J.C.O. 1992
The impressive incremental success since the 1970's has been accomplished by empirical, often flowed clinical trials using chemotherapy agents that singly range in effectiveness from fair to mediocre. This speaks well for the perserverance and inventiveness of the clinical investigators who are responsible for this remarquable success.
Preoperative chemotherapy
In his pilot study preoperative chemotherapy has been used by G. Rosen, as the best way to test cytotoxics and effective dosages on the primitive tumor.
This technics has optimized the treatment of occult metastases by individualization.
Multicentric trials, that tried to "reproduce" Rosen's protocol, have forgotten the analytic thought of this phase and have only retained "the receipt".
Main causes of the low efficacy of multicentric studies
Minimal goals in order to permit inclusion of patients from small centers.
Controlled studies but ignoring main prognostic factors as surgery !, as MTX hydration...
Heavy administration leading to late publications of already known results !
1°) The multicentric trials to verify the effectiveness of chemotherapy in osteosarcoma delayed the systematic use of chemotherapy for 3 or 4 years.
2°) Preliminary results of COSS 77-82 which falsely concluded that amputed patients had more chances of disease free survival than others, delayed the conservative surgery for 5 years. The definitive conclusions of these trials invalidated their preliminary reports.
3°) The superiority of Rosen's protocols has been continuously challenged for 15 years, by randomized studies.
Correlation between DFS and MTX D I
The fundamental value of high dose methotrexate became evident by macro-analysis of all published trials on this subject.
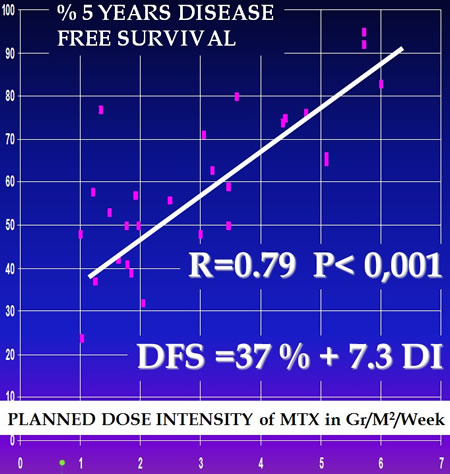 Macroanalysis of osteosarcoma trials. Nicole Delépine and all. Cancer, 08/1996
Macroanalysis of osteosarcoma trials. Nicole Delépine and all. Cancer, 08/1996
Conclusion
In osteosarcoma multicentric randomized trials led always to worse results than pilot studies performed by experimented teams.
Multicentric randomized trials of the last 25 years have not been effective for patients with osteosarcoma.
Even they have been harmful to them !
Multicentric randomized trials have rarely been helpful to the knowledge of the illness.
Their high costs (linked to the heavy organisation) are not justified by their direct results.
Multicentric randomized trials served only to convince reticent medical community of the necessity of chemotherapy in osteosarcoma.
In the same time, many patients let their limb or/and their life because of these bad trials.
Side utilities of randomized studies :
- Regular meetings between doctors from different countries and specialities.
- Revision of pathologic slides.
- International registers.
Isn't possible to obtain these marginal effects with a lower cost ?
Grant-aided meetings
Central register
etc...
Conclusion ot the COSS trials :
- It is necessary to increase the dosage of MTX.
- Adriamycin is a main drug.
- CDDP IV = CDDP IA.
Results of the E.O.I.
- ADR CDDP > HD MTX.
- Combination (60% versus 40%)
- ADR-CDDP = HDMTX (40% versus 40%)
- These results are the same of all protocols that don't use HDMTX.


