Publié sur :
European Musculo Skeletal Tumor Society - 2008
Voir la version PDF de cette publication :

Adjustement of dose in children by routin determination of ifosfamide and its metabolites after the first treatment session.
B. Gourmel, S. Alkahlaf,
Nicole Delepine
Adjustement of dose in children by routin determination of ifosfamide and its metabolites after the first treatment session.
Introduction
Ifosfamide is one of the current reference agents largely used in the treatment of sarcoma. Especially for children it is administered as a continuous infusion.
For this study the drug was administered during 4, 5 or 6 days at the dose of 3g/m²/d and the treatment comprises several courses.
60 patients, over 3 years, aged from 6 to 21 years, and suffering from bone and soft tissue sarcoma were followed during their treatment session.
18 patients were followed over 4 treatment sessions.
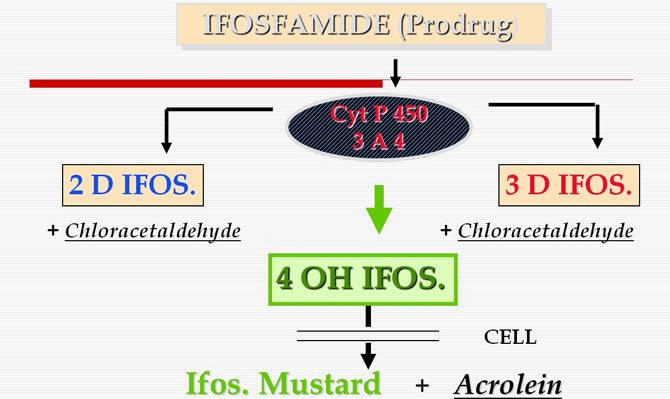
This prodrug is metabolized :
- either by deactivation to produce the dechloroethylated metabolites 2DIf and 3DIf, responsible of the neurotoxicity,
- or by activation with hydroxylation to produce the 4 OH If. split within the cell into acrolein and mustard, the true alkylating agent.
Furthermore the parent compound is capable of auto induction by activating cyt. P-450 (3A4) in liver microsomes.
The goal of this study was to determine if neurotoxicity can be prevented and alkylating potential can be estimated after the first treatment session.
Method
Determination of each compound (Ifos., 2 and 3 dechloro-ifos. and 4 hydroxy-ifos.) was performed using a G.C analytical method, which allows simultaneous detection of the 4 compounds during the same chromatogram.
Blood samples were withdrawn each day: (T0, T12h, T24h, T48h, T72h, T96h and T120h) in special tubes containing the trapping agent.
PK analysis was performed on the basis of Area Under Curve (AUC) for Ifos. and metabolites using Excel Spreadsheet.
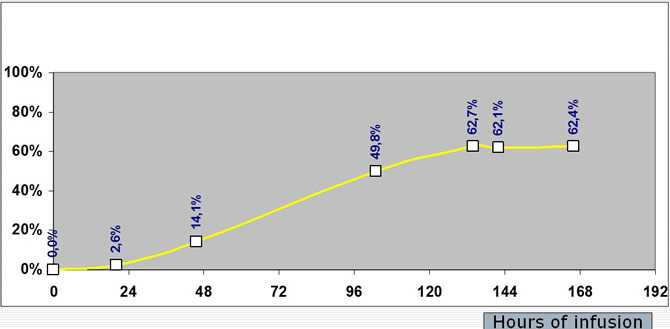
The percentage of induction was estimated for ifos. as blood levels at T24/T120.
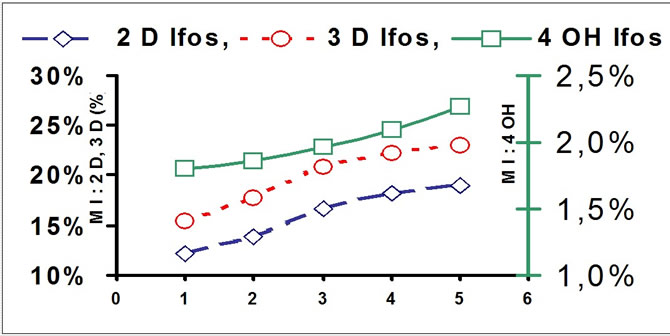
The metabolite index (MI) was : AUC of metabolite divided by AUC of Ifos. (MIday1, day2,... Miday5).
Results (1)
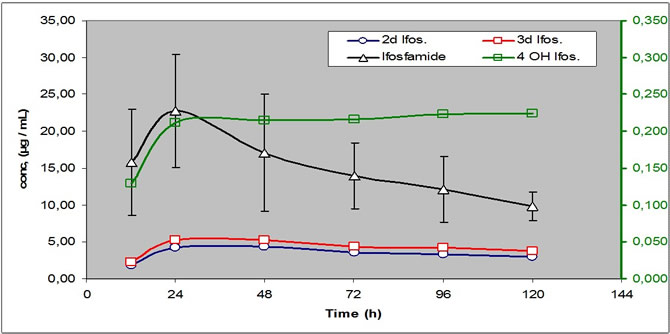
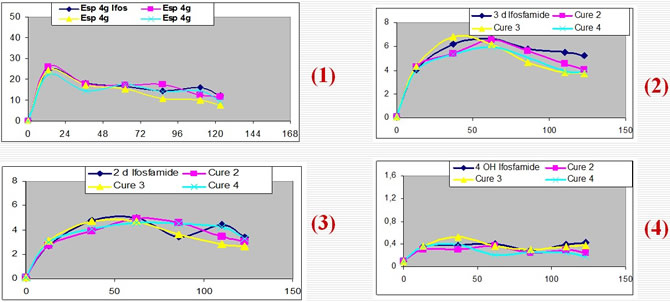
The Area Under Curve (AUC) for ifos. and its metabolites, AUC 0-24, 24-48, were established for each compound and for each treatment session.
Ifos. blood level raised to a maximum at the end of day1, then they regularly decreased to reach a steady state at the end of day 5. The percentage of induction was equal to 48.9% +/-21.2%.
The MI.of 2DIf. and 3D If showed a continuous increase mainly important during the 3 first days and then reach a relative steady state.
The estimation of the MI of the active metabolite 4OHIf showed a more specific increase observed essentially for days 4 and 5.
Pharmacokinetic of Ifos. and its Metabolites during a five days infusion
 Percentage of Ifosfamide induction during a seven days continuous infusion at a dose of 3g/m²/d
Percentage of Ifosfamide induction during a seven days continuous infusion at a dose of 3g/m²/d
 Metabolite Index of 2d Ifos., 3d Ifos. And 4 Hydorxy Ifos.
Metabolite Index of 2d Ifos., 3d Ifos. And 4 Hydorxy Ifos.
 Inter individuals Variability
Inter individuals Variability
These results represent the averages of 60 patients. A large variability inter individuals was obseved.
Nevertheless some patients present a different way of metabolism.
In some cases the production of dechlorinated compounds are higher than the calculated values.
In other cases the production of these metabolites are in the normal range, but the level of the active 4 Hydroxy is lower than the estimated averange.
In these conditions what is the effect on these patients of the successives cures?
Results (2)
The 4 global profiles obtained for Ifos. showed a similar shape.
The statistical comparison demonstrated that no significant difference existed between treatment sessions 1 to 4, either for the blood levels than the estimation of the percentage of induction.
The daily estimation of the MI for inactive metabolites showed a continuous increase during the infusion duration.
It was observed for any of the treatment session. No significant differences were demonstrated for the variation of MI during the infusion whatever treatment session was considered.
Final Values of the Metabolite Index during the 4 consecutive treatment sessions
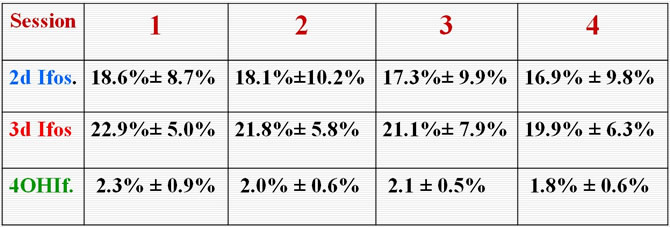 Pharmacokietics of Ifosfamide (1), 2 and 3 Dechloro ethyl Ifosfamide (2, 3) and 4 Hydroxy Ifosfamide (4) over 4 consecutive treatment sessions
Pharmacokietics of Ifosfamide (1), 2 and 3 Dechloro ethyl Ifosfamide (2, 3) and 4 Hydroxy Ifosfamide (4) over 4 consecutive treatment sessions
Consequently in the case of successive treatment sessions, for a given patient, no significant differences were demonstrated for the variation of MI during the infusion whatever treatment session was considered.
At the end of the first infusion, calculation of the MI index informs us on the comportment of the patient toward the drug, provided an identical session is applied (dose, duration and associated drugs).
We can then estimate the risk of neurotoxycity and on the other way the alkylating potential witch will occure in the next sessions.
Conclusion
This study demonstrated a significant induction process of Ifosfamide administred to children at the dose of 3g/m²/d during 4, 5 or 6 days.
Each metabolite index is shown to increase during the global duration of the induction, mainly due to a decrease of the Ifos blood level. The mean values have been calculated and serve as reference.
In the case of consecutive cures the statistical comparison demonstrated that no significant difference existed between treatment sessions 1 to 4.
In these conditions a similar shape to the first kinetic will be observed for a given patient for the next treatment sessions.
At the end of the first pharmacokinetic we calculate the MI of each patient.
Potential risk of neuro-toxicity can be minimized by a reduced dose for the next course if the MI is superior to the reference value calculated previously.
Inversely low MI of the active metabolite without increase of the toxic metabolites can be followed by an increase of the dose.
This method is now applied routinely and no major toxicity have been noted.


