Publication PowerPoint Nicole Delépine - 2009
Voir la version PDF de cette publication :

Original, generic medicines and copy II
Dr Alkhallaf, Dr H Cornille, Dr
Nicole Delepine
Original, generic medicines and copy
"what difference between the unique human we are and the human we could be : our true twin, our false twin, our brother, our sister, our cousin ? The same for generics ?"
The market of the generic medicines in EU
The market of the generic medicines in EU represented 7 billion euros on a total of 70 billion euros of the pharmaceutical market in 2004.
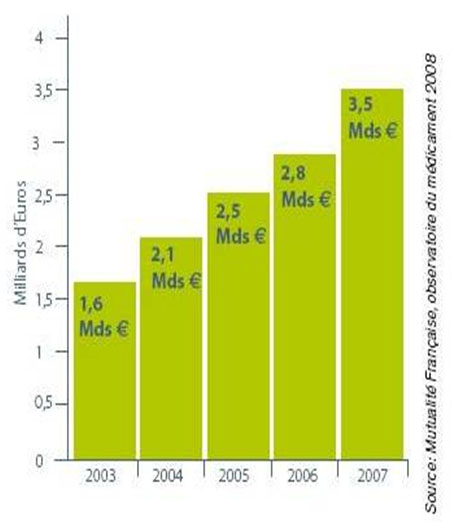
-Countries with a market exceeding 40 %: Denmark, Germany, Holland, Poland, UK
-Market < 20 %: Austria, Belgium, France, Italy, Portugal and Spain.
-40 % for the USA
"Generic products reduce healthcare expenditure and create market competition, and it is broadly assumed that these drugs are identical to the original branded reference drug product"
History of the generic medicine
The notion of generic medicine appeared in the 50s.
Only 30 years later, appeared the first definition in France
It is the committee of the competition (notice of 1981) that expresses:
" We say by generic medicine any copy of an original medicine among which the production and the marketing are made possible, in particular by the fall of certificates in the public domain once sold the legal period of protection "
Definition
A generic medicine is the copy of an original medicine =( princeps ) the patent of which fell
A laboratory which discovers a molecule made a patent and keeps it for approximately 20 years.
After 20 years , the other laboratories can copy the original medicine called : a generic medicine.
Why a generic medicine cheaper that an original ?
The cost price of a generic medicine is much lower than the original medicine, only because of the expenses of the "envelope" of the molecule (the science studying this phenomenon is called the galenic) and of the "marketing" that are necessary for its development.
The expenses of research and development were financed by the firm marketing the original medicine.
The generic medicines:
- which have no expenses of R & D
- allow to be approximately 30 % cheaper that the original medicine
Differences and common points between an original and generic medicine
-
Not 100 % identical to the original medicine
-From a chemical point of view the 2 molecules are perfectly alike
-Concerning the effects of the generic medicine some differences can arise :
-the effect can appear more quickly
-it can be more or less important
-the science studying these phenomena is called the pharmacokinetics.
Criteria of safety and efficiency (according to Heller , 2006)
-The pharmaceutical company which produces the original medicine has to state toxicological, pharmacological and clinical studies.
-For the copy, the company has to produce a scientific literature giving evidence of the safety and the efficiency of the product.
-The generic medicines) must be demonstrated as " essentially similar to the original medicine "
-(Directive 2004 / 27 / CE published on March 31st, 2004).
Public health code Art. R 5143-9 (decree of March 13th, 1997)
Bioavailability is:
the speed and the intensity of the absorption in the body
-from a pharmaceutical shape
-from an active principle
-or from its therapeutic fraction,
-intended to become available at the level of the sites of action
bio-equivalence : the equivalence of bioavailabilities.
Therapeutic equivalence of a generic
Bioequivalence or bio-disponibility must be identical to the original
markers of bio-disponibility:
-AUC(area under curve)
-C max (concentration max of the drug)
-T max : time to reach this maximal concentration.
Differences in the estimate point
Data of the generic medicine compared with those of the original medicine
expressed as a relationship between generic vs original
-" the Estimate point "
-A 90 % confidence interval is determined.
-" Estimate point " and its 90 % confidence interval have to lie within an interval from 0.8 to 1.25 .
European and French legislation on the generic medicines
According to the Directive 2004/27, of April 30th, 2004:
-the European recommendation stipulates to " accept a bio-equivalence of the generic medicine situated between 80 % and 125 % of that some reference medicine ".
BUT : for medicines with narrow therapeutic margin:
-As anti-arythmics, anti-epileptics, oral anti-coagulants, digitalics, immunosuppressive drugs, hypoglycemiants, theophylline, etc
the 90 % confidence interval has to be situated between 0.9 and 1.11
so the law is largely insufficient.
Differences in the T max
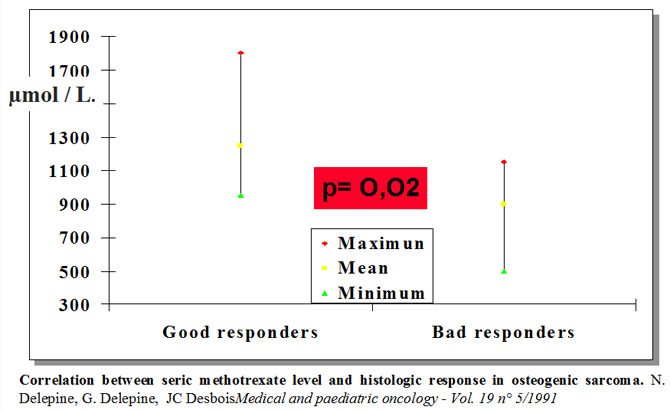
We have observed that mean methotrexatemia durint préoperative phase is coorelated with response.
Of the Tmax among the pharmacokinetic data
The Tmax is the time necessary to reach the maximal concentration
This parameter has less importance when the wanted pharmacological effect is not connected to the speed of action : treatment of HTA , of hypercholesterolemia etc
BUT, for some pathology the pharmacological effect must be quickly obtained :
- painful state, asthma attack, hyperglycemia
The medicine has to act quickly and the Tmax is an essential parameter from this point of view.
Different excipients
Certain excipients that have special effects in certain patients are:
-described " as excipients with notorious effect "
-summed up in a list published by the European Commission (Directive July, 2003).
But, the same active principle can exist in the form of different salts.
the pharmacokinetics can be identical for every salt (eg diclofenac sodic and diclofenac potassium),
But it is not always the case, in particular for example:
-propoxyphène, pilocarpine, lincomycine, penicillin G, alprenolo
various salts can have a different bioavailability.
" Excipient with Notorious Effect " (ENE)
Any excipient the presence of which can require precautions for use for particular patients
Excipients are the only elements which may vary between a princeps and its generic medicines
75 % of the generic medicines contain ENE.
20 % of the generic medicines contain more ENE than the princeps.
Risk of changes of excipients
Excipients can be different : the change of medecine, with possibly a change of excipients, can lead to serious clinical risks.
e.g.: cases of saccharose, some sodium or some potassium which can lead to serious effects for the patient affected by diabetes or by renal pathologies.
Drugs in R & D. 9(2):65-72, March 1, 2008.Genazzani, Armando A; Pattarino, Franco
"In practice, despite legislation demanding demonstration of pharmaceutical equivalence and bioequivalence, thereby ensuring the safety and efficacy of the product, generic products can differ significantly from the reference drug"
particularly in terms of pharmacokinetic properties.
These differences most often relate to pharmaceutics differences in the production of the active principle ingredient
-(e.g. different crystalline forms or particle size)
Or to use of excipients (such as sugars)
Or to the manufacturing process itself (such as tablet manufacture).
Uncertainties on the quality of generic medicines
Punctual study sponsored by ROCHE: 34 generic medicines containing of the ceftriaxone in comparison with Rocéphine Quality criteria:
-standards of the European and American pharmacopoeias and the pharmaceutical standards of ROCHE.
Uncertainties on the quality of generic medicines : results for this example
17 parameters:
including physical aspect contents
purity
presence of toxin
diverse particles etc. were analyzed for these 34 generic medicines
Therapeutic equivalence with the original substance?
10/34 generic medicines did not respect the parameters of American and European quality of pharmacopoeias and 4 were not sterile!
-Regarding the quality standards of the firm ROCHE, no generic medicines respected the totality of the parameters
The most frequent violations :
-clarity of the solution
-presence of tracks of products of degradation
Uncertainties on the quality of generic medicines
The generic medicines have a therapeutic equivalence in the original substance ?
Drugs in R & D. 9(2):65-72, March 1, 2008.Genazzani, Armando A; Pattarino, Franco
"from the patient's perspective, changing from branded to generic drugs can give rise to concerns about the switching. Although sufficient safeguards exist to ensure patient safety and generic drug efficacy,
it should not be assumed that all generics are entirely identical".
Anti-epileptic medicines
Certain generic medicines made in the USA have a lower bioavailability of 30 % as compared to the original medicine after taking a fat meal Spontaneous side effects were reported to lamotrigine treatment in USA
In 14 cases, a loss of the control of the epilepsy was noted.
Investigation realized by 6920 American neurologists:
The passage of the original substance to the generic medicine leaded to :
-the arisen of epileptic crisis up to 67.8 %
-an increase of the side effects of 56 %
In conclusion
Attention to excipients, coloring or other, being able to lead to an allergic reaction.
The addition of an excipient or a change in a complex composition in excipients can raise problems especially in the case of chronic diseases well treated with the principal medecine.
The replacement can be at the origin of confusions.
Risk of changes of excipients
the medicines are different in spite of having the same DCI (international common denomination) as the medicine originally prescribed !
Patients and even doctors may not be careful!!!
PRECAUTIONS
Avoid the replacement for certain categories of population in particular the old and\or allergic persons.
Avoid the replacement with medicines with narrow therapeutic margin.
Avoid the replacement between generic medicines.
Conclusion and awareness
If you must absolutely use generics.
Try not to switch.
Initiate the treatment with the generics.
For elderly patients try to conserve the initial branded medicine or the generics they usually use.


